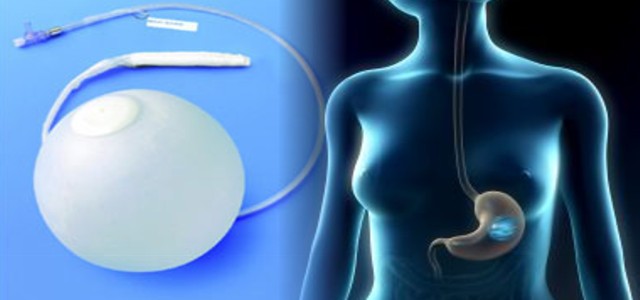
Intragastric Balloon
Cosmetic Division
What is Intragastric Balloon?
Intragastric balloon is a relatively new medical device intended for effective body mass loss. It has become widely known during the recent years. It is used for obesity treatment and intended for the patient’s weight loss by means of partial filling of the stomach and, as a result, the absence of hunger. The set consists of a collapsed silicone balloon with a check valve, a silicone probe for the balloon placement with a silicone sheath, a metal guide wire for increased rigidity, a PVC tube for liquid transfusion.
The gastric balloon procedure (endoscopic intragastric balloon) leaves an inflated silicon balloon in the stomach for 6 months, making less room for food. As a result, patients:
- Feel full sooner while eating and therefore eat less
- Lose about 30% of their excess weight in 6 months
- Have health improvement for diabetes, joint/bone disease, and heart-related issues

How does it work?
Intragastric balloon is a spherical shell made of durable silicone with a check-valve attached to its wall, and a system of tubes for saline solution transfusion. The collapsed balloon is placed inside the stomach as a gastric probe, without any liquid inside. In the process of the balloon inflation with saline solution, the balloon becomes sphere-shaped. According to the initial volume of stomach, the size of the inflated balloon varies from 400 to 700 cc. When inside the stomach, the balloon fills the stomach excess capacity. Consequently, less food is needed to induce the reaction of receptors sending signals about the stomach filling. Thus, the patient feels satiety earlier than usual. With the course of time, the patient works out the habit to consume less food. Then the patient accustoms himself to the new diet and nutrition regime which is maintained for a long period after the balloon removal. The patient suffers from nausea, stomach overfilling, discomfort in the epigastrium area during the first several days after the balloon placement. But normally, within 7-14 days after intragastric balloon placement procedure, the patient almost doesn’t make notice of the device and lives the normal life style. The balloon is placed temporarily. The balloon can stay in the stomach for maximum 6 months and should be removed afterwards.
The advantages over other methods
• The balloon implantation procedure is not regarded as surgery. The balloon is implanted endoscopically which takes 10-15 minutes, under intravenous anesthesia;
• The procedure is tolerated easily by patients and can be compared with regular endoscopy;
• The patient doesn’t have to change his usual lifestyle;
• High efficiency;
• Minor risk of complications
Safety
The decision to use the balloon is made by a physician on the basis of the data obtained as a result of the total examination of a patient suffering from obesity. The balloon can be placed into the stomach of patients whose body mass index (BMI) is 30-40 kg/m2. Patients whose BMI is more than 40 kg/m2 can be treated with the balloon for the purpose of preparation for the surgery.
Contraindications
• Any inflammatory disease of the gastrointestinal tract including esophagitis, gastric ulceration, duodenal ulceration, Crohn’s disease.
• Gastrointestinal tract cancer
• Esophageal or gastric varices, telangiectasis.
• Congenital anomalies of the gastrointestinal tract such as atresias or stenoses
• A stricture or diverticulum of the esophagus or pharynx
• Prior gastric or intestinal surgery
• A large hiatal hernia
• Psychological disorder, alcoholism or drug addiction
• Pregnancy or breast-feeding (if pregnancy is confirmed at any time during the course of balloon treatment, the device should be removed)
• Patients receiving aspirin, anti-inflammatory agents, anticoagulants or other gastric irritants
• Allergic reaction to silicone
• Any other medical condition which would not permit endoscopy
• It is not recommended to use the device for patients with low discipline unwilling to participate in an established diet and behavior modification program.




